Why We Still Don’t Understand the Gut Microbiome
In a nutshell
We Still Can’t Define a “Healthy” Gut Microbiome - despite decades of research and thousands of peer-reviewed publications
Stool Samples Don’t Represent the Whole Gut – poor research starting material
Widespread Use of Better Sampling Methods is Essential
I’ve spent the past few years trying to understand the human microbiome and its role in my health. My first three articles - written after I shared how malnutrition contributed to my heart disease - focused on the gut microbiome:
Since then, I’ve changed my mind about how confidently we can describe the human body's relationship with its gut microbes. While decades of research and thousands of papers have revealed much about this complex ecosystem, there are still some fundamental things we simply don’t know.
Where We Are Now?
We do know that environmental factors - diet, sunlight, urban vs. rural living, pharmaceuticals, and even cleaning products - affect the structure and function of our microbiome.
But three recent eye-opening publications remind us what we don’t know:
Microbial diversity may not correlate with gut health - 2017: Diversity is the question, not the answer [1]
Still no solid definition of a “healthy microbiome - 2024: Examining the healthy human microbiome concept [2]
Whether microbiome changes are even necessary for health benefits - 2025: The microbiome: An actor or stage for biotic interventions? [3]
A quote from the 2025 paper really struck me:
“The raison d’être for developing biotic interventions is to deliver a positive health outcome to the host. At the moment, we cannot say that a change in the microbiome is key to providing those benefits.”
In this post, I want to explain what I believe is the biggest obstacle holding us back: how we’re studying the gut microbiome. I’ll cover the following:
Structure of the human gut and how it shapes its microbiome
The big problem: We’re studying the wrong thing
Why this matters
I’ve drawn from the eleven scientific publications listed below for this article. When it mattered, I referenced individual papers.
Let’s look at what shapes the microbes inside us.
Structure of the Human Gut and how it shapes its microbiome
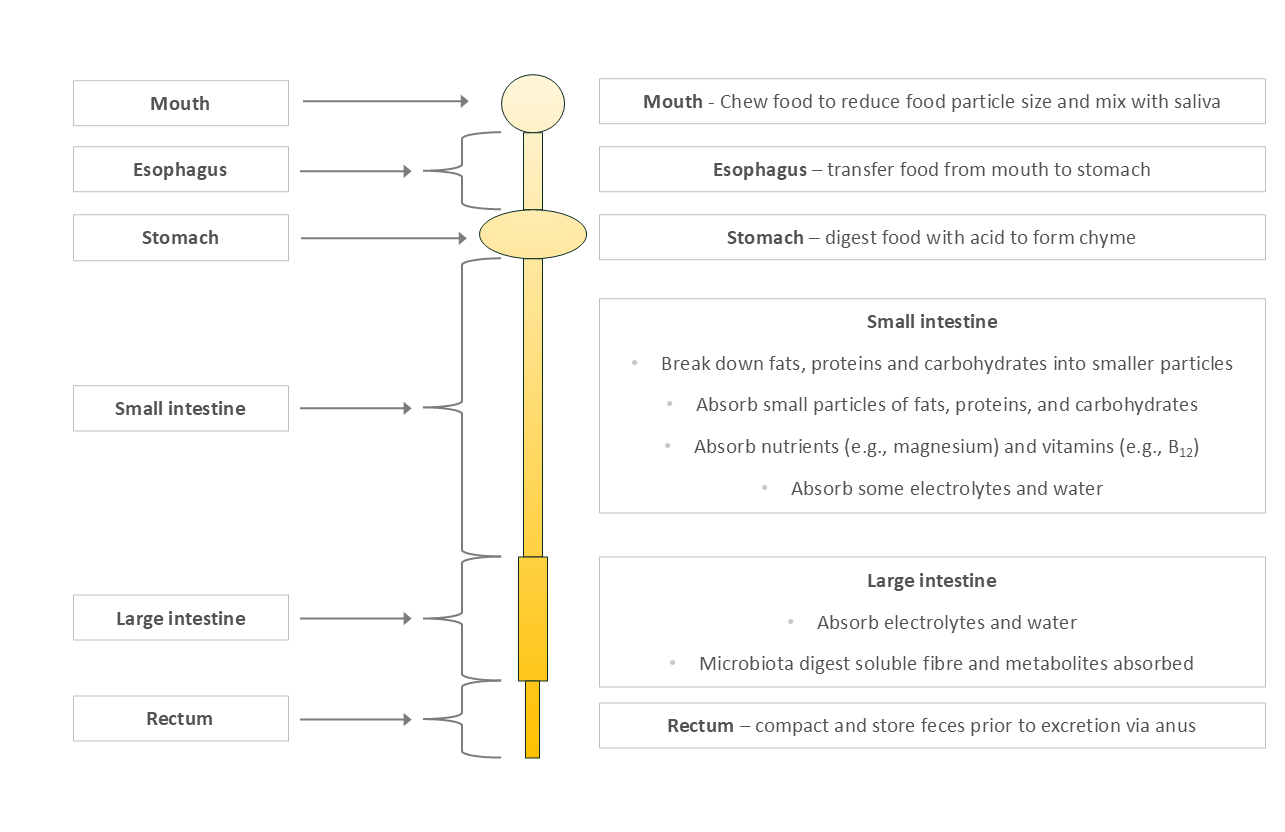
Humans evolved on a diet high in digestible proteins, fats, and simple carbohydrates, and relatively low in fibrous plant matter. Our digestive system (Figure 1) reflects that evolutionary history:
Small, acidic stomach
Long small intestine - where most nutrient absorption happens
Short large intestine - where most fermentation occurs
Figure 1: Simplified Human Digestive System
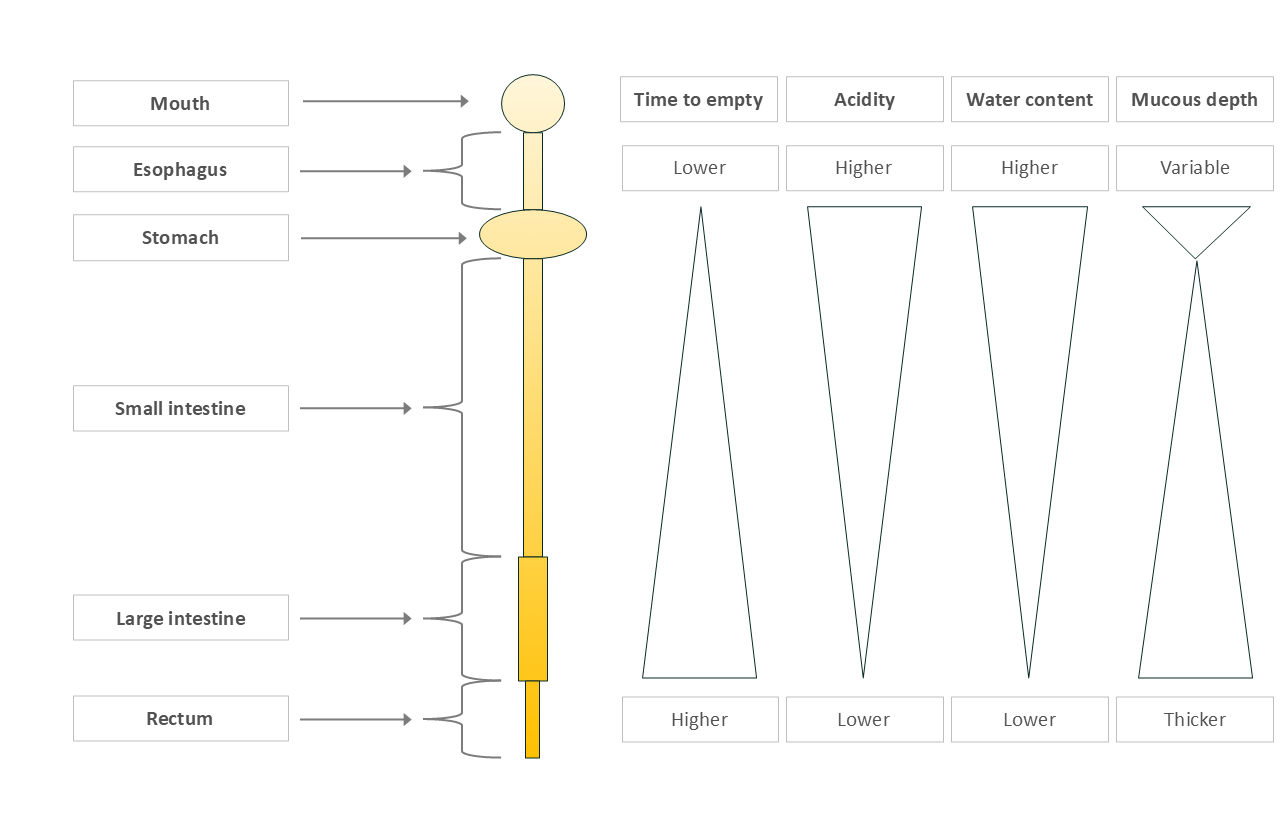
Physical features of the human gut that Influence our microbes
Transit time: Food moves quickly through the stomach and small intestine, then slows in the large intestine, where most fermentation happens.
Acidity: The stomach is highly acidic, protecting us from pathogens. Acidity decreases along the digestive tract.
Water content: High at the beginning to aid digestion and absorption, then reabsorbed later to conserve water.
Mucus layers: Mucus lines the gut to protect us from stomach acid and our own microbes. The stomach (very acidic) and large intestine (greatest number of microbes) have two layers; the small intestine just one.
Figure 2: Physical characteristics influencing microbiota composition
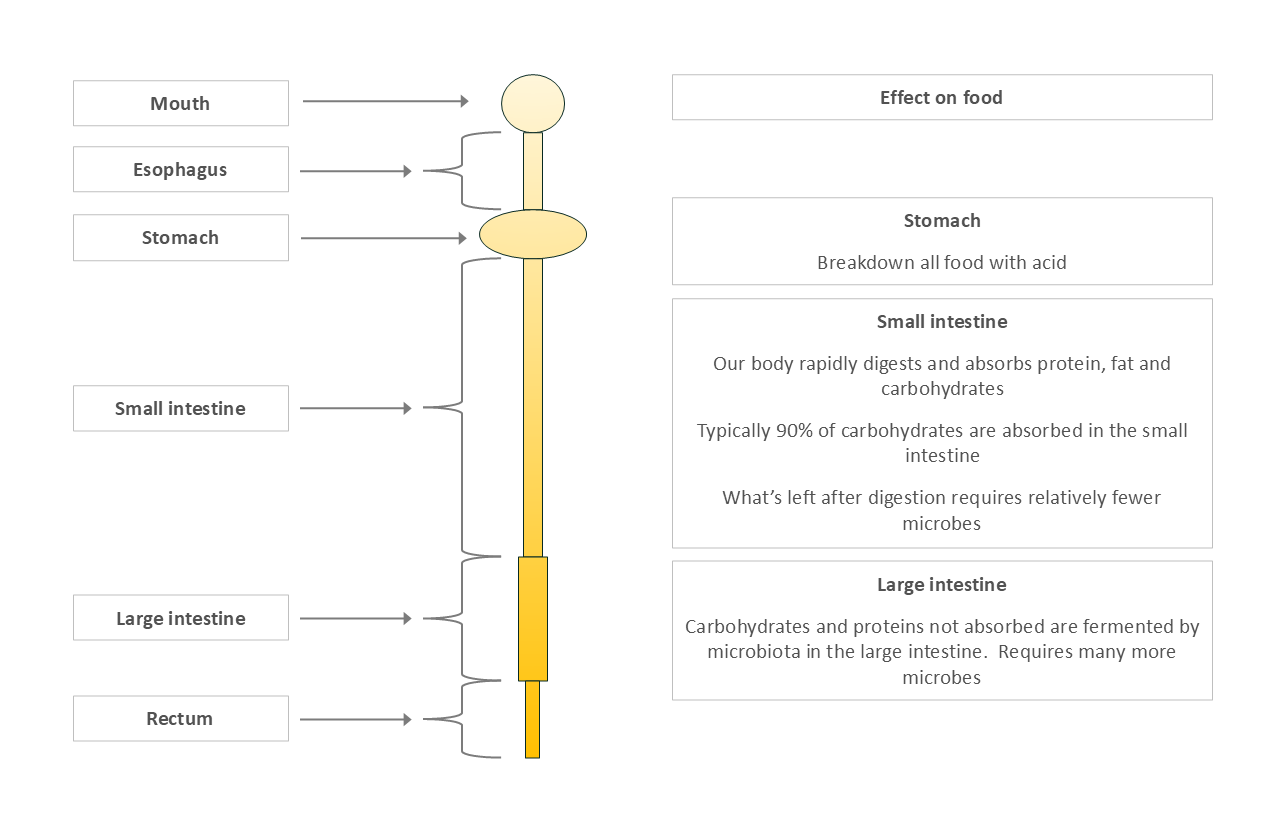
Our digestion creates a nutritional gradient along the gut
Stomach: High acidity, minimal absorption (except things like alcohol).
Small Intestine: Where 90% of human digestion and nutrient absorption happens. Not designed to house microbes for complex fermentation.
Large Intestine: Designed to handle undigested plant fiber, excess protein, and mucous from the small intestine - fuel for extensive microbial fermentation.
The complex carbohydrates that make it to the large intestine require many different microbes working together to break them down. This results in the high microbial numbers and high diversity described below.
Figure 3: Nutrient Availability in the Gut
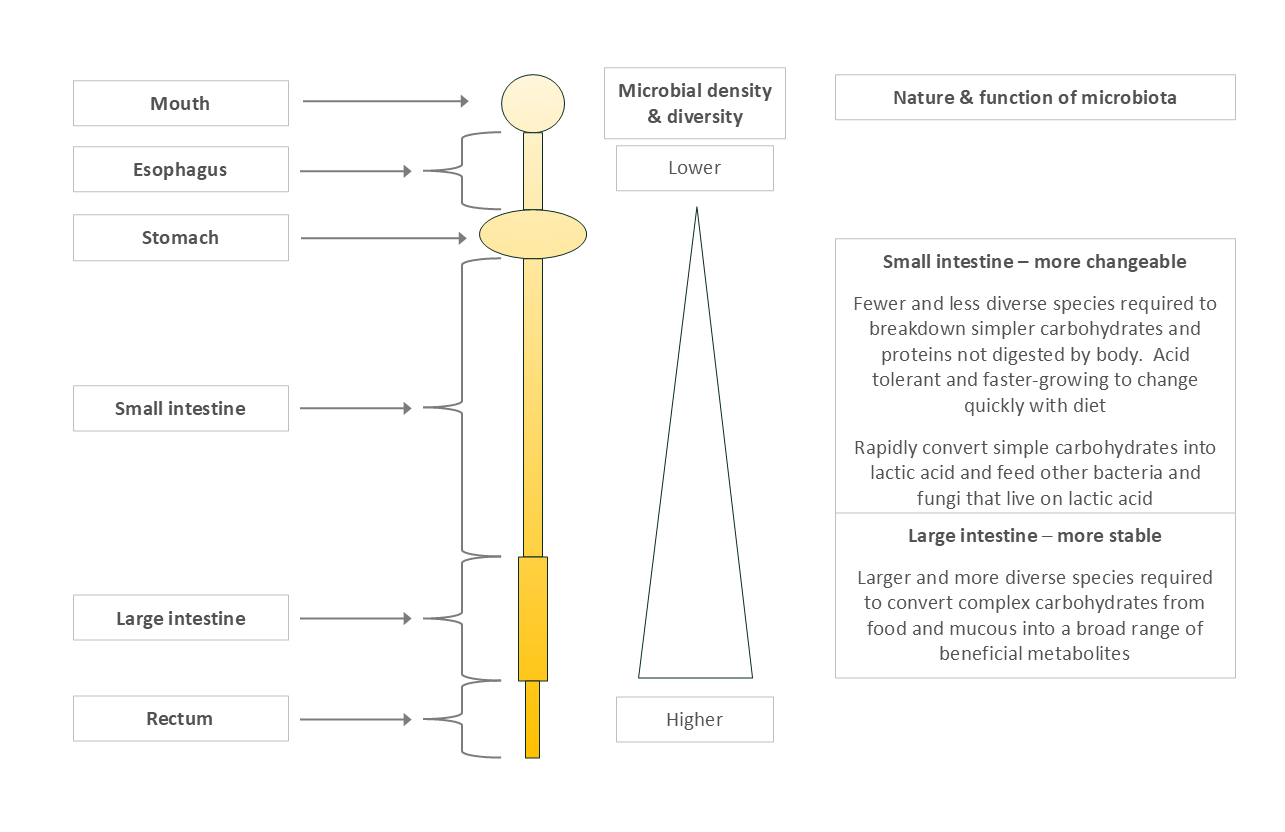
Microbial Distribution Along the Gut
Microbial numbers and diversity increase along the gut (Figure4):
Stomach & upper small intestine: Low microbial presence due to acidity and limited food for them.
Lower small intestine: A bit more diversity, but still relatively limited.
Large intestine: Microbial heaven—high fiber, mucus from the small intestine, slow transit, complex fermentation, and nutrient sharing.
The large intestine is where the “heavy lifting” of fermentation occurs, requiring large, diverse, and cooperative microbial communities.
Figure 4: Microbial Type and Load by Gut Region
The Big Problem: We’re Studying the Wrong Thing
Despite its known complexity, most gut microbiome research still relies on the wrong starting material: stool samples.
Here’s the problem: we haven’t (yet) demonstrated that stool represents the entire gut. At best, it tells us what’s happening at the very end – it likely represents the rectum, and maybe the large intestine, although we can’t confidently declare the latter yet.
Why Stool Sampling Falls Short
Microbiologist Jelena Vulevic recently summed up the limitations well:
Misrepresentation of Gut Microbiota Diversity: Stool samples primarily reflect the distal colon and rectum, representing only a small part of the gut. This approach misses the significant microbial diversity in the small intestine, where most nutrient absorption and immune signalling occur.
Mucosal vs. Luminal Microbiota: Stool largely captures luminal bacteria, while the more clinically relevant mucosa-associated microbiota are often overlooked. Mucosal microbiota are closely associated with the gut barrier and immune system, making them critical for understanding host-microbe interactions.
Sampling and Intra-Sample Variability: Stool is just the final, excreted portion of a long and complex digestive system. Microbial communities vary significantly along the GI tract, making a single stool sample a poor proxy for the full gut ecosystem.
Transit Time and Selection Pressure: Only bacteria that can survive the entire digestive process are present in stool, potentially missing key microbial players from earlier in the GI tract.
Disconnection from the Host Context: Stool samples provide limited insights into host-microbe interactions, gut barrier function, and immune signalling, which are critical for understanding health and disease.
A 2023 study using ingestible sampling devices [5] concluded:
“…stool provides neither a complete nor accurate representation of the longitudinal and temporal variability of the microbiota composition, virus activity, host proteome, and bile acid contents within the intestines.”
Thankfully, better internal sampling methods are being developed [6]. Unfortunately, their adoption is not yet widespread.
The Orchestra Analogy
Studying only stool is like listening to an orchestra from outside the concert hall. You’ll hear muffled music, but miss the nuance - the interaction between instruments, the conductor’s cues, and the individual musicians.
You’ll leave intrigued, maybe even moved. But you won’t understand why it sounded the way it did. And you definitely won’t be able to predict what a different combination of musical instruments will sound like.
That’s what we’re doing with the gut microbiome right now.
Why This Matters
This is not just an academic puzzle, as the 2025 review warns [3]:
“While it is tempting to assume that the specific composition of the microbiome …[of]…a healthy individual is itself healthy, it could in fact…be predisposing the host to health issues in later life. Equally, intervening to change the microbiome of an unhealthy individual runs the risk of worsening a condition rather than alleviating it or could result in exposing the host to other unknown risks of future ill health.”
The ancient principle of “First, do no harm” should guide every biotic intervention and every microbiome-related health recommendation.
We’re not there yet.
Summary
We’ve learned a lot about the stool microbiome - but we’re still in the early days of understanding the gut microbiome. The gut is an incredibly complex ecosystem. Its microbial inhabitants vary by location, time, food, and many other individual factors.
Stool samples may give us clues - but far from the full picture.
If we truly want to understand how the microbiome affects human health, we need to start looking inside the gut with the right tools, at the right places, at the right time.
Finally, we need to be humble enough to admit what we still don’t know.
References
Shade, A. Diversity is the question, not the answer. ISME J 11, 1–6 (2017). https://doi.org/10.1038/ismej.2016.118
Joos, R., Boucher, K., Lavelle, A. et al. Examining the healthy human microbiome concept. Nat Rev Microbiol 23, 192–205 (2025). https://doi.org/10.1038/s41579-024-01107-0
Sanders ME, Hill C. The microbiome: An actor or stage for the beneficial action of probiotics, prebiotics, synbiotics, and postbiotics? Cell Host Microbe. 2025 Jun 11;33(6):777-789. doi: 10.1016/j.chom.2025.04.017. PMID: 40505618.
Zoetendal EG, Raes J, van den Bogert B, Arumugam M, Booijink CC, Troost FJ, Bork P, Wels M, de Vos WM, Kleerebezem M. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012 Jul;6(7):1415-26. doi: 10.1038/ismej.2011.212. Epub 2012 Jan 19. PMID: 22258098; PMCID: PMC3379644
Shalon, D., Culver, R.N., Grembi, J.A. et al. Profiling the human intestinal environment under physiological conditions. Nature 617, 581–591 (2023). https://doi.org/10.1038/s41586-023-05989-7
Small intestine vs. colon ecology and physiology: Why it matters in probiotic administration Jensen, Benjamin Anderschou Holbech et al. Cell Reports Medicine, Volume 4, Issue 9, 101190
Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013 Jun;10(6):352-61. doi: 10.1038/nrgastro.2013.35. Epub 2013 Mar 12. PMID: 23478383; PMCID: PMC3758667.
Renate A A A Ruigrok, Valerie Collij, Paula Sureda, Marjolein A Y Klaassen, Laura A Bolte, Bernadien H Jansen, Michiel D Voskuil, Jingyuan Fu, Cisca Wijmenga, Alexandra Zhernakova, Rinse K Weersma, Arnau Vich Vila, The Composition and Metabolic Potential of the Human Small Intestinal Microbiota Within the Context of Inflammatory Bowel Disease, Journal of Crohn's and Colitis, Volume 15, Issue 8, August 2021, Pages 1326–1338, https://doi.org/10.1093/ecco-jcc/jjab020
Procházková N, Falony G, Dragsted LO, Licht TR, Raes J, Roager HM. Advancing human gut microbiota research by considering gut transit time. Gut. 2023 Jan;72(1):180-191. doi: 10.1136/gutjnl-2022-328166. Epub 2022 Sep 28. PMID: 36171079; PMCID: PMC9763197
Benjamin Anderschou Holbech Jensen, et al Small intestine vs. colon ecology and physiology: Why it matters in probiotic administration, Cell Reports Medicine, Volume 4, Issue 9, 2023
Shalon, D., Culver, R.N., Grembi, J.A. et al. Profiling the human intestinal environment under physiological conditions. Nature 617, 581–591 (2023). https://doi.org/10.1038/s41586-023-05989-7